Nanofibers are defined as fibrous materials with a diameter of 1 nm to 100 nm and a length of 100 times or more the diameter. Through various research and development efforts, a wide variety of nanofibers have been produced to date, both from natural and synthetic materials. Natural nanofibers (bio-nanofibers) include polymers such as cellulose nanofibers (CNF), silk fibroin, collagen, gelatin, keratin, chitosan, and alginate. DNA is also a type of bio-nanofiber. Synthetic polymers include polypropylene (PP), polyethylene terephthalate (PET), polylactic acid (PLA), polycaprolactone (PCL), and polyurethane (PU). Development is also progressing on other materials such as carbon nanotubes (CNT) and metal nanowires.
Nanofibers have a wide range of physical properties and potential applications. First, because they have an extremely large surface area per unit volume and are highly porous, it is possible to create materials with excellent hydrophilicity, hydrophobicity, and molecular adsorption properties. Active researches are also underway into new functions that utilize the quantum size effect (Kubo effect), in which energy does not exist continuously as in bulk materials but changes into discrete units, and the unique optical, electrical conductivity, and thermal properties that arise from supramolecular assemblies formed by van der Waals and coordinate bonds. Technologies that have already been put to practical use include catalysts and antibacterial materials that utilize the high adsorption and molecular recognition properties of a large specific surface area, sensors, battery materials, electronic materials, scaffolding materials and bio-immobilization carriers in regenerative medicines, high-performance filters that combine gas permeability and high collection efficiency by utilizing the slip-flow effect of microporous materials, and films and fiber materials that utilize optical properties.


There are various processes for producing nanofibers depending on the material. Electrospinning, which applies high voltage to a polymer solution, and conjugate melt spinning, a molten polymer extrusion technique developed using a spinneret, are used to produce synthetic polymers such as polyamide, polyurethane, and polylactic acid. Naturally derived nanofibers such as cellulose nanofiber (CNF) can be manufactured by combining mechanical and chemical processes. Mechanical processing involves mechanically defibrating raw materials such as wood and pulp in a wet process. Specifically, mechanical defibration is carried out using a stone grinder, ball mill, bead mill, high-pressure homogenizer, etc. In addition to mechanical defibration, auxiliary chemical treatments such as acid treatment and enzymatic decomposition may be used. Carbon nanotubes (CNTs) are produced using chemical vapor deposition (CVD), arc synthesis, laser ablation, etc.
There are two methods for collecting and storing nanoparticles and nanofibers: dry and wet. Dry methods are technically difficult in terms of the level of sealing, filter collection performance, and safety exposure measures, and a standard process has not yet been established. Wet processes allow the raw materials to be contained in a liquid or gel, which helps to control dispersion and exposure risks, and are therefore used in many nanofiber manufacturing and distribution processes.


If the fiber length is short and the aspect ratio is close to that of nanoparticles, it can be dried and granulated using a spray dryer and distributed as a granulated product. Nanofibers with long fiber length tend to aggregate and are difficult to return to their original state even when resuspended in liquid, whereas short fiber length has a high proportion of crystalline structure, making it easy to redisperse the dried product in liquid. This makes it possible to distribute the product in a dry state, which reduces transportation costs.
Cellulose nanofiber (CNF)
Cellulose is a polymer fiber bundle made up of molecular chains found in plants, and is existed in large quantities in nature. Nanocellulose is a material made by defibrating and refining cellulose fibers to nano-size. Among nanocelluloses, those with long fibers are called cellulose nanofibers (CNF), while those with short fibers and a high proportion of crystalline structure are called cellulose nanocrystals (CNC). Nanocellulose is an environmentally friendly material because it is derived from plants and fixes CO2. In terms of physical properties, it is about one-fifth the weight of steel and five times stronger. It also has other characteristics such as high water absorption, low thermal expansion, and excellent transparency.
Cellulose nanofibers are usually distributed as aqueous or gel dispersions. Unlike cellulose nanocrystals (CNCs) with a high crystalline structure that can be spray-dried, cellulose nanofibers with a high aspect ratio (long fiber length) tend to aggregate strongly during drying, making them difficult to redisperse in liquids and resulting in a loss of their properties.
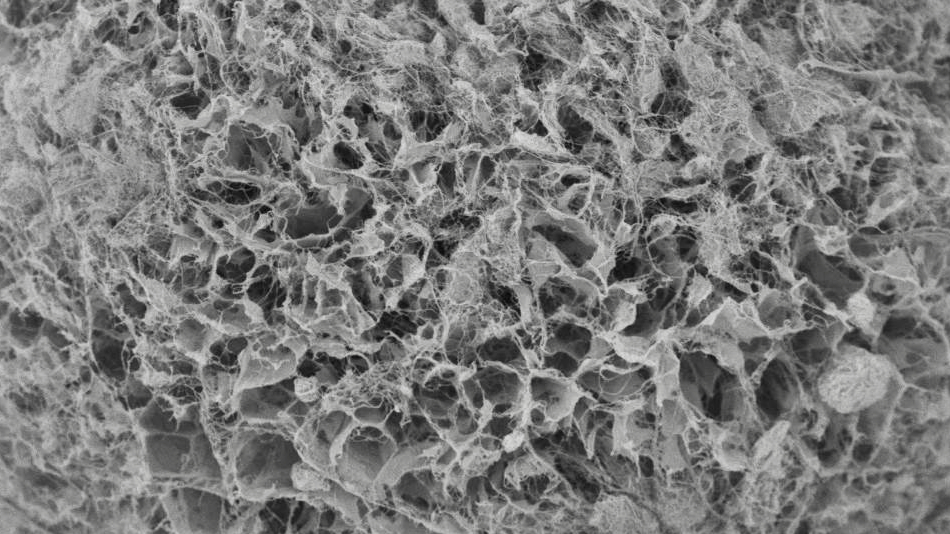
To evaluate the re-dispersibility of cellulose nanofiber, we produced dry powder using spray drying and freeze granulation processes, and conducted a comparative study of re-dispersibility.


The granules in the photos were produced by processing 2.0 wt% cellulose nanofiber dispersion in freeze granulation. Experimental results showed that the granules retained their nanofiber structure. This is thought to be due to the extremely minimal movement of nanofibers during freeze drying process of the frozen bodies produced using the freeze granulator. Whereas spray drying causes the granules to shrink and aggregate, freeze granulation can produce solid granules with a homogeneous internal structure.
Cellulose nanofibers are environmentally friendly and have many advantages in terms of physical properties. In addition to the fields in which they have been put to practical use to date, such as reinforcing materials and water absorbents, they are expected to be used as composite materials for a wide range of applications, including cosmetics, clothing, food additives, packaging materials, optical materials, and more.
* A patent application for this result is currently being filed jointly by PRECI and Daio Paper Corporation.
Carbon nanotube (CNT)
Carbon has the most covalent bonds of any element, with four pairs, and serves as the framework for creating various molecules. It also has a variety of forms and allotropes, ranging from soft substances such as graphite to diamond, the hardest natural substance. Products made from the element include activated carbon, carbon black, and carbon fibers. At the nano level, carbon allotropes have structures such as graphene, a sheet-like substance with a hexagonal lattice structure, C60 fullerene, which has a soccer-ball-like structure consisting of 60 carbon atoms, and carbon nanotubes (CNTs), which are tube-shaped graphene sheets. After the CVD synthesis method for CNTs was invented by Professor Morinobu Endo of (then) Shinshu University in 1976, the structure of single-walled CNTs was elucidated by Sumio Iijima of (then) NEC Fundamental Research Laboratories in 1993.
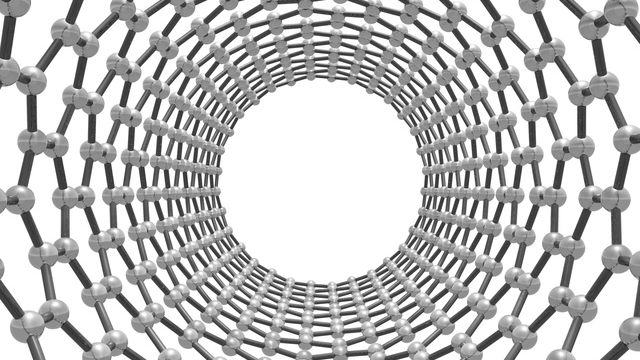
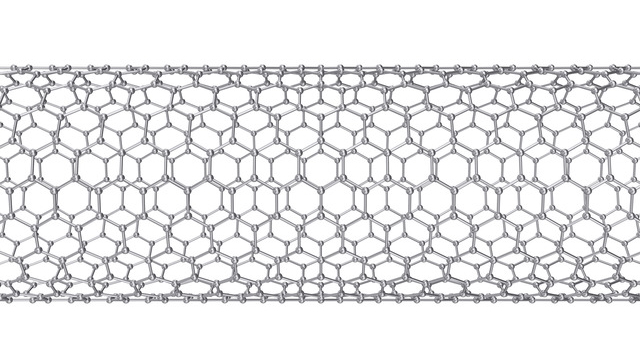
Carbon nanotubes (CNTs) are broadly divided into single-walled CNTs and multi-walled CNTs. Single-walled CNTs have a diameter of approximately 1 – 4 nm and are said to be 20 times stronger than steel, 10 times more thermally conductive than copper, and 1,000 times more electrically conductive than copper. Single-walled CNTs also have high chemical reactivity, and these properties have led to their increasing use primarily in the field of high-performance materials. Multi-walled CNTs have a diameter of approximately 4 to 150 nm, are highly productive, and are inexpensive. Carbon nanofibers are also a type of multi-walled CNT. Depending on their structure, CNTs can be given conductive or semiconductive properties.
Carbon nanotubes (CNTs), like cellulose nanofibers (CNFs), tend to aggregate and are difficult to disperse in water or organic solvents. Furthermore, the longer the tube length, the greater the tendency for aggregation, resulting in a decrease in functional properties. Similarly, when it comes to CNT dried powder produced by spray drying, the powder can strongly be agglomerated during the drying process, significantly reducing its specific surface area and making it difficult to redisperse in liquid. As with the case of cellulose nanofiber, we are conducting research to improve redispersibility by producing dried CNT granules using our world’s first mass-production freeze granulator.



We not only provide powder processing trials for spray drying, spray cooling, and freeze granulation, but also services that include pre- and post-powder processing, such as wet pulverizing, mixing, molding, sintering and freeze-drying. We operate a total of three locations: two Powder Technical Centers in Japan and ASEAN Powder Technical Center in Thailand. Our brand new Powder Technical Center 2 (PTC2), which was newly established in 2023, has one of the largest collections of analytical measurement equipment in Japan. We provide one-stop support for powder processing and analytical measurements (Powder Trials & Analytical Measurements/Contract Powder Processing).
*The contents such as photos shown in this article may differ from the actual projects and may be used as an images.